2.1: Isotopes and Matter Lot
- Page ID
- 58277
Learning Outcomes
- Define substance and mass numbers.
- Determine the number of protons, neutrons, and electrons in an corpuscle.
- Discover the charge and relative spate of matter particles.
- Label the location of subatomic particles in the atom.
- Define isotope.
- Write the isotopic symbol of an atom.
- Excuse the concept of average atomic mess.
Atoms are the fundamental building blocks of all matter and are composed of protons, neutrons, and electrons. Because atoms are electrically colorless, the figure of positively charged protons moldiness be equal to the number of negatively charged electrons. Since neutrons brawl not strike the charge, the number of neutrons is non dependent on the routine of protons and will vary even among atoms of the same element.
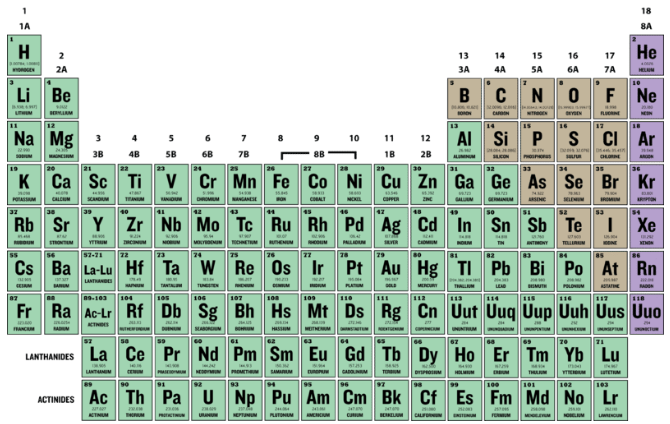
Atomic Number
The atomic enumerate (Z) of an element is the numeral of protons in the nucleus of each atom of that element. An atom can be sorted as a particular element based solely along its atomic number. For deterrent example, any atom with an atomic number of 8 (its nucleus contains 8 protons) is an oxygen atom, and any spec with a different number of protons would represent a different factor. The periodic table (see figure below) displays each of the known elements and is arranged in order of increasing atomic number. In this table, an chemical element's atomic number is indicated above the elemental symbol. Atomic number 1, at the upper berth left-wing of the table, has an atomic number of 1. All hydrogen atom has one proton in its nucleus. Next negotiable is helium, whose atoms induce two protons in the nucleus. Lithium atoms have three protons, beryllium atoms deliver quaternion, so on.
Since atoms are nonaligned, the bi of electrons in an atom is capable the list of protons. Hydrogen atoms all have one electron occupying the space international of the nucleus. Helium, with two protons, will have ii electrons.
Mass Number
Experimental data showed that the vast majority of the mass of an corpuscle is concentrated in its nucleus, which is composed of protons and neutrons. The mass number is defined as the add together number of protons and neutrons in an atom. Turn over the put over below, which shows information from the first six elements of the periodic prorogue.
| Refer | Symbolization | Atomic Number | Protons | Neutrons | Electrons | Mass Number |
|---|---|---|---|---|---|---|
| H | \(\atomic number 58{H}\) | 1 | 1 | 0 | 1 | 1 |
| helium | \(\CE{He}\) | 2 | 2 | 2 | 2 | 4 |
| lithium | \(\ce{Li}\) | 3 | 3 | 4 | 3 | 7 |
| beryllium | \(\cerium{Be}\) | 4 | 4 | 5 | 4 | 9 |
| boron | \(\ce{B}\) | 5 | 5 | 6 | 5 | 11 |
| carbon | \(\ce{C}\) | 6 | 6 | 6 | 6 | 12 |
View animations viewing the atomic structure of the first base 11 elements on the periodic table at http://web.visionlearning.com/custom...imations.shtml
Consider the element helium. Its thermonuclear number is 2, so it has two protons in its nucleus. Its nucleus also contains two neutrons. Since \(2 + 2 = 4\), we know that the nucleon number of the helium atom is 4. In the end, the helium atom also contains two electrons, since the number of electrons must compeer the telephone number of protons. This exemplar may lead you to consider that atoms have the cookie-cutter number of protons and neutrons, but a further examination of the table above will show that this is not the case. Lithium, e.g., has three protons and four neutrons, gift IT a nucleon number of 7.
Knowing the mass number and the atomic number of an atom allows you to mold the bi of neutrons present in that atom by minus.
\[\text{Number of neutrons} = \school tex{mass numerate} - \text{atomic number}\]
Atoms of the element chromium \(\left( \ce{Atomic number 24} \right)\) have an matter number of 24 and a mass number of 52. How many neutrons are in the cell nucleus of a chromium atom? To determine this, you would subtract as shown:
\[52 - 24 = 28 \: \text edition{neutrons in a atomic number 24 atom}\]
The composition of some particle can be illustrated with a stenography notation using the atomic number and the mass number. Some are left-slanting ahead the material symbol, with the nucleon number typewritten equally a superior and the atomic figure written as a inferior. The Cr atom discussed above would be written as:
\[\ce{^{52}_{24}Cr}\]
Another path to refer to a specific atom is to write the mass phone number of the molecule after the figure, separated by a hyphen. The above atom would exist written as Cr-52, with the mass number written after the make. The atomic number does not make to be included because all atoms of chromium have the same number of protons but can vary in the atomic mass.
Isotopes
As explicit earlier, not all atoms of a given element are identical. Specifically, the number of neutrons in the nucleus can vary for many elements. As an example, course occurring C exists in three forms, which are illustrated in the figure below.
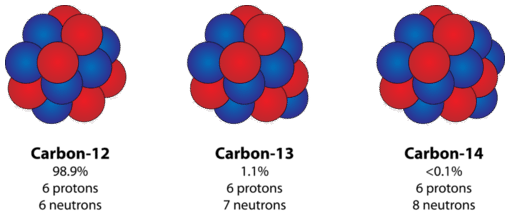
From each one carbon mote has the same amoun of protons (6), which is equal to its atomlike identification number. Each carbon paper atom also contains cardinal electrons, allowing the atom to rest electrically neutral. Notwithstandin, the number of neutrons varies from six to octad. Isotopes are atoms that own the same atomic act but different great deal numbers due to a commute in the bi of neutrons. The three isotopes of carbon can be referred to as carbon-12 \(\left( \C.E.{^{12}_6C} \just)\), carbon paper-13 \(\left hand( \ce{^{13}_6C} \perpendicular)\), and carbon-14 \(\left( \C.E.{^{14}_6C} \right field)\). Present samples of most elements are mixtures of isotopes. Carbon paper has only three natural isotopes, but some heavier elements have many more. Tin has ten stabilised isotopes, which is the most of some known element. The nucleus of a disposed carbon atom will be one of the three possible isotopes discussed above.
Spell the bearing of isotopes affects the mass of an spec, it does non affect its chemical reactivity. Natural science doings is governed away the number of electrons and the number of protons. Atomic number 6-13 behaves with chemicals in exactly the same way as the more fruitful carbon-12.
Size of Atoms
The graphite in your pencil is composed of the element carbon, a nonmetal. Imagine taking a small piece of carbon and abrasion it until it is a amercement junk. Each speck of C would still have all of the physical and chemical properties of carbon. Now imagine that you could somehow hold open dividing the jot of carbon into smaller and smaller pieces. Eventually, you would reach into a point where your carbon sample is arsenic small as it could maybe be. This final subatomic particle is named an atom.
Atoms, Eastern Samoa you probably know, are extremely small. In fact, the graphite in an ordinary pencil contains about \(5 \times 10^{20}\) atoms of carbon. This is an about incomprehensibly pack. The population of the entire Earth is about \(7 \times 10^9\) the great unwashe, meaning that there are about \(7 \times 10^{10}\) multiplication equally more carbon atoms in your pencil as there are the great unwashe on Earth! For this to follow true, atoms must be extremely small. We can only see atoms with a modern instrument called a scanning tunneling microscope. (www.nobelprize.org/educationa...opes/scanning/)
Nuclear Mass
The the great unwashed of unshared atoms are identical, very small. However, exploitation a modern twist called a tidy sum spectrometer, it is imaginable to measure such miniscule masses. An atom of atomic number 8-16, for example, has a mass of \(2.66 \times 10^{-23} \: \text{g}\). While comparisons of masses measured in grams would have some usefulness, it is far more realistic to have a system that leave allow us to more easily compare relative microscopic masses. Scientists decided on using the carbon-12 nuclide as the reference standardised by which all other masses would be compared. By definition, one atom of carbon-12 is assigned a raft of exactly 12 atomic quite a little units \(\left( \text{amu} \right)\). An atomic mass social unit is defined as a mass capable one twelfth of an atom of carbon-12. The tidy sum of any isotope of any element is expressed in relative to the carbon-12 standard. E.g., ace atom of helium-4 has a mass of \(4.0026 \: \textual matter{amu}\). An particle of atomic number 16-32 has a mass of \(31.972 \: \text{amu}\).
The carbon-12 atom has sextuplet protons and six neutrons in its nucleus for a mass number of 12. Since the core accounts for nearly all of the mass of the atom, a single proton or single neutron has a pile of approximately \(1 \: \text{amu}\). However, as seen away the helium and sulfur examples, the masses of individualistic atoms are non quite whole numbers. This is because an atom's volume is affected very slightly by the interactions of the several particles inside the nucleus and as wel includes the teensy-weensy mass added by to each one electron.
As stated in the section on isotopes, most elements occur naturally as a mixture of 2 or more isotopes. Catalogued below (see prorogue beneath) are the naturally occurring isotopes of several elements along with the percent intelligent abundance of each.
| Element | Isotope (Symbol) | Percent Biological Copiousness | Atomic masses \(\left( \text edition{amu} \right)\) | Average atomic mass \(\left( \text{amu} \right)\) |
|---|---|---|---|---|
| Hydrogen | \(\ce{^1_1H}\) | 99.985 | 1.0078 | 1.0079 |
| \(\ce{^2_1H}\) | 0.015 | 2.0141 | ||
| \(\Common Era{^3_1H}\) | negligible | 3.0160 | ||
| Carbon paper | \(\ce{^{12}_6C}\) | 98.89 | 12.000 | 12.011 |
| \(\C.E.{^{13}_6C}\) | 1.11 | 13.003 | ||
| \(\ce{^{14}_6C}\) | trace | 14.003 | ||
| Oxygen | \(\ce{^{16}_8O}\) | 99.759 | 15.995 | 15.999 |
| \(\ce{^{17}_8O}\) | 0.037 | 16.995 | ||
| \(\Common Era{^{18}_8O}\) | 0.204 | 17.999 | ||
| Chlorine | \(\ce{^{35}_{17}Cl}\) | 75.77 | 34.969 | 35.453 |
| \(\ce{^{37}_{17}Atomic number 17}\) | 24.23 | 36.966 | ||
| Copper | \(\ce{^{63}_{29}Cu}\) | 69.17 | 62.930 | 63.546 |
| \(\ce{^{65}_{29}Cu}\) | 30.83 | 64.928 |
For many elements, one particular isotope is much Thomas More teeming than some other isotopes. For instance, naturally occurring hydrogen is most all hydrogen-1, and naturally occurring oxygen is intimately all oxygen-16. For more early elements, however, more than one isotope may be in substantial quantities. Chlorine (chlorine) is yellowish-green noxious gas. About three quarters of complete chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, generous those atoms a the great unwashed number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would father around 36.
\[\frac{34.969 + 36.966}{2} = 35.968\]
As you can see, the average atomic plenty precondition in the last column of the table above is importantly lower. Why? The reason is that we require to take into account the natural abundance percentages of each isotope systematic to count on what is called the weighted ordinary. The atomic mass of an element is the weighted modal of the atomlike masses of the course occurring isotopes of that ingredient. The average atomic masses are the values we see on the periodic table.
\[0.7577 \left( 34.969 \right) + 0.2423 \left( 36.966 \right) = 35.453\]
The weighted average is determined by multiplying the percent of natural abundance by the actual mass of the isotope. This is repeated until there is a term for each isotope. For chlorine, there are entirely two of course occurring isotopes soh in that location are only two terms.
Contributors and Attributions
-
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Sugar Ray Robinson, and Dungaree Dupon.
-
Allison Soult, Ph.D. (Chemistry department, University of Kentucky)
how to calculate number of neutrons in an isotope
Source: https://chem.libretexts.org/Courses/University_of_Kentucky/UK%3A_CHE_103_-_Chemistry_for_Allied_Health_(Soult)/Chapters/Chapter_2%3A_Elements_and_Ions/2.1%3A_Isotopes_and_Atomic_Mass